1,2,3-tribromopropane
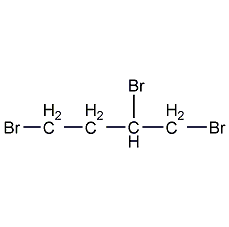
Structural formula
| Business number | 02AF |
|---|---|
| Molecular formula | C3H5Br3 |
| Molecular weight | 280.78 |
| label |
tribromopropane, s-Tribromopropane, sym-Tribromopropane, BrCH2CH(Br)CH2Br, nematicides, Halogenated hydrocarbon solvents, aliphatic compounds |
Numbering system
CAS number:96-11-7
MDL number:MFCD00017884
EINECS number:202-478-8
RTECS number:TZ8300000
BRN number:1732082
PubChem number:24848905
Physical property data
1. Properties: colorless or light yellow liquid, irritating
2. Density (g/mL, 20/4℃): 2.4209
3. Relative density (25℃, 4℃): 2.4107
4. Melting point (ºC): 76.2
5. Boiling point (ºC, normal pressure): 220
6 . Refractive index at room temperature (n25): 1.5836
7. Refractive index (n20ºC): 1.5862
8. Flash point (ºC): 94
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure ( mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil-water (octanol/water) partition coefficient relationship Value: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: soluble in ethanol, ether and chloroform, insoluble in water
Toxicological data
1. Acute toxicity: Rat oral LDLo: 500mg/kg;
2. Reproductive toxicity
Rat oral TDLo: 250mg/kg (male rats 5 days old before mating); rat intraperitoneal TDLo: 23869 μg/kg (male rats 1 day before mating);
3. Mutagenicity
Microbial Salmonella typhimurium mutation: 1 μmol/plate ;
Microbiological Salmonella typhimurium mutation: 500μg/plate;
Transperitoneal DNA damage in rats: 1404μg/kg;
DNA damage in rat testicles : 1μmol/L;
Rat oral dominant lethal test: 250mg/kg/5D; Toxic, harmful if inhaled or taken.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 39.10
2. Molar volume (cm3/mol): 117.9
3. Isotonic specific volume (90.2K): 305.3
4. Surface tension (dyne/cm): 44.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 15.50
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 25.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxidants. Avoid contact with skin as it may cause irritation.
Storage method
Store in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. It should not be stored in large quantities or for long periods of time. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Preparation method:
![]()
In a reaction bottle equipped with a stirrer, reflux condenser (equipped with a calcium chloride drying tube), dropping funnel, and thermometer, add 182g (1.5mol) of allyl bromide ① (2) ), 250mL dry carbon tetrachloride. Cool to -5°C in an ice-salt bath, add 255g (1.6 mol) of dry bromine dropwise from the dropping funnel, and control the dropping speed to raise the reaction solution to 0°C, and complete the addition in about 1.5 hours. Slowly warm to room temperature and continue stirring for 30 min. The solvent was distilled under reduced pressure, and then the fraction at 92-93°C/1.33kpa was collected to obtain 400g of almost colorless liquid 1,2,3-tribromopropane (1), with a yield of 95%. Note: ① Allyl bromide is best treated before use. The treatment method is as follows: first dry with anhydrous calcium chloride, then distill, and collect the fraction at 69~72°C. [1]
Purpose
Used in nematicides, solvents, and organic synthesis intermediates.
extended-reading:https://www.newtopchem.com/archives/1845extended-reading:https://www.morpholine.org/high-quality-n-dimethylaminopropyldiisopropanolamine-cas-63469-23-8-n-3-dimethyl-amino-propyl-n-n-diisopropanolamine/extended-reading:https://www.cyclohexylamine.net/sponge-hardener/extended-reading:https://www.bdmaee.net/cas-2273-43-0/extended-reading:https://www.bdmaee.net/tertiary-amine-composite-catalyst/extended-reading:https://www.bdmaee.net/benzyldimethylamine/extended-reading:https://www.bdmaee.net/dabco-tetn-catalyst-cas280-57-9-evonik-germany/extended-reading:https://www.newtopchem.com/archives/39970extended-reading:https://www.bdmaee.net/tegoamin-bde-catalyst-cas121-54-0-degussa-ag/extended-reading:https://www.bdmaee.net/niax-catalyst-a-400/

