1H-benzotriazole
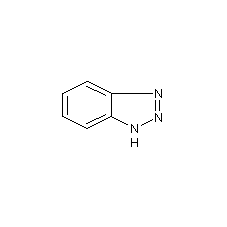
Structural formula
| Physical competition number | 028N |
|---|---|
| Molecular formula | C7H5N3 |
| Molecular weight | 119.12 |
| label |
phenyltriazole, 1H-benzotriazole, Benztriazole, benzotriazepine, Indyne, benzotriazole, 1,2,-aminozophenylene, 1,2,3-triaza-1h-indene, 1,2,3-Benzotriazole, BTA, corrosion inhibitor, Heterocyclic compounds |
Numbering system
CAS number:95-14-7
MDL number:MFCD00005699
EINECS number:202-394-1
RTECS number:DM1225000
BRN number:112133
PubChem number:24847810
Physical property data
1. Properties: White to light brown needle-like crystals. Odorless. It oxidizes in the air and gradually turns red. Can explode when distilled in vacuum.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 98.5
5. Boiling point (ºC, normal pressure): 204
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 170
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure ( kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, benzene, toluene, chloroform and dimethyl Formamide, slightly soluble in cold water. Insoluble in petroleum solvents.
Toxicological data
1. Acute toxicity: Rat oral LD50: 560mg/kg; Rat inhalation LD50: 1910mg/m3/24H; Rat skin contact LD50: >1mg/kg; Mouse oral LC: 615mg/kg; Mouse abdominal LC50: 400mg/kg; Mouse intravenous LC50: 238mg/kg; Rabbit skin contact LDLo: 450mg/kg; Guinea pig oral LD50: 500mg/kg;
2. Other multiple-dose toxicity: rat inhalation TDLo: 109mg/kg/26W-I;
3. Chronic toxicity/carcinogenicity
Rat Oral TDLo: 220mg/kg/78W-I; Mouse oral TDLo: 770mg/kg/78W-I;
4. Mutagenicity
Mutation of microorganism Salmonella typhimurium : 100μg/plate;
E. coli mutation: 33300μg/plate;
Rat embryonic morphological transformation: 94μg/plate;
5. Acute toxicity:
Oral LD50 560mg/kg(rat)
Skin LD50 615mg/kg(mus)
>2000mg/kg(rbt)
Inhalation LC50/4H 1400mg/m3/4H(rat)
Irritation to eyes severe 100mg(rbt)
Main irritating effects:
On the skin: General products will irritate the skin
On the eyes: Irritating effects
Sensitization: None Known sensitization
Ecological data
Slightly harmful to water. Strongly irritating to the eyes and moderately irritating to the skin.
Molecular structure data
1. Molar refractive index: 34.71
2. Molar volume (cm3/mol): 88.3
3. Isotonic specific volume (90.2K ): 259.0
4. Surface tension (dyne/cm): 73.9
5. Polarizability (10-24cm3): 13.76
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 41.6
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 92.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides.
2. Toxic, oral MLD500mg/kg, intraperitoneal injection of LD501000mg/kg, intravenous injection of LD50238mg/kg in rats. The equipment should be sealed and operators should wear protective equipment.
Storage method
1. Store sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep sealed. Keep away from sources of fire and store away from oxidizing agents.
2. Lined with plastic bags and packaged in wooden barrels, or in glass bottles. 500g per bottle, 20 bottles packed in a wooden box. “Light-proof” and “Sealed” are marked on the outside of the box. During storage and transportation, prevent moisture, sun protection, heat insulation, keep dry and well ventilated.
Synthesis method
1. Obtained from the reaction of o-phenylenetriamine and sodium nitrite. Add o-phenylenediamine to 50°C water to dissolve, then add glacial acetic acid, cool to 5°C, add sodium nitrite and stir the reaction. The reactant gradually turns dark green, the temperature rises to 70-80°C, and the solution turns orange. Leave it at room temperature for 2 hours, cool, filter out the crystals, wash with ice water, and dry to obtain a crude product. Distill the crude product under reduced pressure and collect 201 The fraction at -204°C (2.0kPa) is recrystallized with benzene to obtain a product with a melting point of 96-97°C, with a yield of about 80%. It has been reported that the by-product benzotriazole was obtained by treating the condensation wastewater of carbendazim with sodium nitrite.
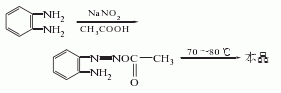
2.O-phenylenediamine diazotization method. Diazotize o-phenylenediamine, and then cyclize it in acetic acid to obtain crude benzotriazole, which is then refined by recrystallization and vacuum dried to obtain a relatively pure benzotriazole.
3.O-phenylenediamine method The o-phenylenediamine method is the most classic method for synthesizing benzotriazole. Including o-phenylenediamine atmospheric pressure synthesis method and improved o-phenylenediamine high-pressure method.
①O-phenylenediamine normal pressure method: First dissolve o-phenylenediamine in acetic acid aqueous solution, and prepare about 40% sodium nitrite aqueous solution. The two solutions are pre-cooled to 1 to 5°C, mixed and reacted, and kept in an ice bath. Then the temperature is quickly raised to 80°C to close the ring to form benzotriazole. After cooling, filter and wash with water to obtain the crude product. Distill under an absolute pressure of 2000 Pa, collect fractions between 2.0 and 204°C, and crystallize with benzene to obtain the product, with a yield of 70% to 80%. Another production example is: add o-phenylenediamine to a 50°C aqueous solution to dissolve, then add glacial acetic acid, cool to 5°C, add sodium nitrite to stir the reaction, the reactant gradually turns dark green, and the temperature rises to 70~80°C. , the solution turns orange-red, leave it at room temperature for 2 hours, cool, filter out the crystals, wash with ice water, and dry to obtain a crude product; distill the crude product under reduced pressure, collect the fractions at 201~204°C (2.0kPa), and then recrystallize with benzene , a product with a melting point of 96 to 97°C can be obtained, with a yield of about 80%.
② The molar ratio of o-phenylenediamine and sodium nitrite in the o-phenylenediamine high-pressure method is 1: (1~1.05), the reaction temperature is 200~300℃, and the pressure is 4.8×106~6.9×106Pa. After the reaction is completed, adjust the pH value to 6 with acid. Since there is no acid involved in the diazotization ring-closure reaction, the chance of producing dark-colored tar from diazo coupling is reduced, thereby increasing the yield of the product and making the purification of the product easier. For example, o-phenylenediamine and 37% sodium nitrite aqueous solution, at a temperature of 260°C and a pressure of 3.0×106~3.3×106Pa.After reacting for 3 hours, cool and use concentrated sulfuric acid to adjust the Pa value from 11.7 to 6 to obtain benzotriazole with a purity of 100% and a yield of 96.9%.
4.Benzimidazolone method Benzimidazolone and sodium nitrite aqueous solution react at 190℃ and high pressure for 75 minutes. After acidification, water washing and drying, the product was obtained with a yield of 85.3%.
5.O-nitrophenylhydrazine method o-Nitrophenylhydrazine is in a mixed aqueous solution of ammonia, isopropyl alcohol and hexylene glycol, React at 140°C and high pressure for 1.5 hours to generate 1-hydroxybenzotriazole (HBTA). Use copper-chromium trioxide as a catalyst, introduce hydrogen and nitrogen in a ratio of 92:8, and perform a deoxygenation and hydrogenation reaction at 160 to 170°C and high pressure for 1 hour. HBTA is deoxygenated and hydrogenated to generate BTA, and finally benzotriazole is recovered. The rate is 89%. The reaction formula is as follows:
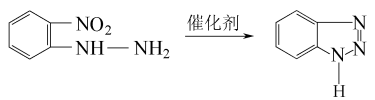
6.The o-nitrochlorobenzene method first directly synthesizes HBTA from o-nitrochlorobenzene and hydrazine hydrate, and then deoxygenates and hydrogenates Generate BTA, with the highest overall yield reaching 8.6%. The advantages of this method are high yield and few intermediate links. It is a promising and important method.
Purpose
1. When combined with ammonium hydroxide and ethylenediaminetetraacetic acid, it can selectively determine silver, copper, and zinc. Photographic anti-fogging agent. Photographic inhibitors. Organic Synthesis.
2.Benzotriazole has an anti-corrosion effect on metal materials such as copper, aluminum, cast iron, nickel, and zinc. It can be used with a variety of corrosion inhibitors, such as chromate, polyphosphate, molybdate, silicate, nitrite, ATMP, HRDP, EDTMP, etc., to improve the corrosion inhibition effectFruit. It has excellent corrosion inhibition effect in closed circulation cooling water system. It can be used in conjunction with a variety of scale inhibitors and bactericidal algaecides. Benzotriazole does not interfere with the corrosion inhibition effect of polyphosphate and has strong resistance to oxidation. However, when it exists at the same time as free chlorine, it loses its corrosion inhibition effect on copper, and after the disappearance of chlorine, its corrosion inhibition effect is restored. Its usage concentration is generally 1 to 2 mg/L, and its corrosion inhibition effect is very good in the pH range of 5.5 to 10, but when the pH value is low The corrosion inhibition effect is reduced in the medium.
3.Added into epoxy resin adhesive to prevent corrosion and blackening of copper and its alloys. The reference dosage is 0.2%~0.5%. For water-soluble adhesives, the adding amount is 0.005% to 0.1%. It is also widely used as anti-rust agent for copper, silver, zinc, aluminum, cast iron and other metals, water purifier, anti-fogging agent in the photographic industry, ultraviolet absorber, paint additive, preservative for synthetic detergents, anticoagulant, lubrication Oil additives, synthetic dye intermediates, polymer material stabilizers, plant growth regulators, anti-discoloration agents, gas phase corrosion inhibitors, antistatic and automotive antifreeze additives, etc.
4.Widely used for corrosion inhibition and rust prevention of copper and silver equipment. It can also be used for the preparation of photographic anti-fog, anti-fog, and gas-phase rust inhibitors.
5.Benzotriazole does not interfere with the corrosion inhibition effect of polyphosphate and has strong resistance to oxidation. . However, when it exists with free chlorine at the same time, it loses its corrosion inhibition effect on copper. After the chlorine disappears, its corrosion inhibition effect is restored. This is a property that MBT cannot have.
6.Used as metal anti-rust and corrosion inhibitor, anti-tarnish agent for copper and silver, and photographic anti-fogging agent.
extended-reading:http://www.newtopchem.com/”>extended-reading:https://www.newtopchem.com/archives/42998extended-reading:https://www.bdmaee.net/dabco-eg-catalyst-cas280-57-9-evonik-germany/extended-reading:https://www.morpholine.org/polyurethane-catalyst-1028/extended-reading:https://www.bdmaee.net/niax-catalyst-a-1/extended-reading:https://www.bdmaee.net/dabco-ne1070-catalyst-cas31506-43-1-evonik-germany/extended-reading:https://www.newtopchem.com/archives/40368extended-reading:https://www.newtopchem.com/archives/40226extended-reading:https://www.bdmaee.net/niax-a-440-delayed-tertiary-amine-catalyst-momentive/extended-reading:https://www.newtopchem.com/archives/1915

