4-aminomethylbenzoic acid
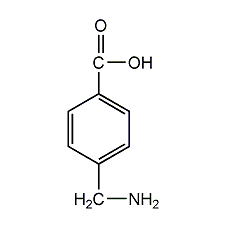
structural formula
| business number | 0184 |
|---|---|
| molecular formula | c8h9no2 |
| molecular weight | 151.17 |
| label |
p-aminomethylbenzoic acid |
numbering system
cas number:56-91-7
mdl number:mfcd00010203
einecs number:200-297-9
rtecs number:none
brn number:1100606
pubchem number:24846170
physical property data
1. character: hydrate it is white scaly or crystalline powder. odorless, slightly bitter taste.
2. density (g/ml,25/4℃) : undetermined
3. relative vapor density (g/ml,air=1): undetermined
4. melting point (ºc): 340-350
5. boiling point (ºc,normal pressure): undetermined
6. boiling point (ºc,5.2kpa): undetermined
7. refractive index: not ok
8. flash point (ºc): undetermined
9. specific optical rotation ( º): undetermined
10. spontaneous ignition point or ignition combustion temperature (ºc): not ok
11. vapor pressure (kpa,25ºc): undetermined
12. saturated vapor pressure ( kpa,60ºc): undetermined
13. heat of combustion (kj/mol): undetermined
14. critical temperature (ºc): undetermined
15. critical pressure (kpa): undetermined
16. oil and water (octanol /water) partition coefficient pair value: undetermined
17. explosion upper limit (%,v/v): not ok
18. lower explosion limit (%,v/v): undetermined
19. solubility: soluble in boiling water, slightly soluble in water, almost insoluble in ethanol, benzene and chloroform.
toxicological data
none yet
ecological data
none yet
molecular structure data
5. molecular property data:
1. molar refractive index: 41.63
2. molar volume (m3/mol):121.9
3. isotonic specific volume (90.2k):335.2
4. surface tension (dyne/cm):57.1
5. polarizability(10-24cm3):16.50
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 2
3. number of hydrogen bond acceptors: 3
4. number of rotatable chemical bonds: 2
5. number of tautomers: none
6. topological molecule polar surface area 63.3
7. number of heavy atoms: 11
8. surface charge: 0
9. complexity: 139
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
this product should be kept sealed.
synthesis method
there are three processes: (1) is derived from the catalytic hydrogenation of p-cyanobenzoic acid; (2) aminated from p-chloromethylbenzoic acid and get; (3) is derived from the amination of p-bromomethylbenzoic acid.
purpose
organic synthesis intermediates. hemostatic drugs and fibrinolytic inhibitors are suitable for various diseases caused by excessive fibrinolytic enzyme activity.
extended-reading:https://www.newtopchem.com/archives/category/products/page/40extended-reading:https://www.bdmaee.net/fascat4400-tertiary-amine-catalyst-arkema-pmc/extended-reading:https://www.bdmaee.net/nt-cat-a-4-catalyst-cas8001-28-0-newtopchem/extended-reading:https://www.bdmaee.net/dabco-ne300-dabco-foaming-catalyst-polyurethane-foaming-catalyst-ne300/extended-reading:https://www.morpholine.org/efficient-reaction-type-equilibrium-catalyst-reactive-equilibrium-catalyst/extended-reading:https://www.newtopchem.com/archives/39736extended-reading:https://www.newtopchem.com/archives/category/products/page/107extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/polyurethane-rigid-foam-catalyst-cas-15875-13-5-catalyst-pc41.pdfextended-reading:https://www.newtopchem.com/archives/40032extended-reading:https://www.cyclohexylamine.net/2-2-dimethylaminoethylmethylamino-ethanol-nnn-trimethylaminoethylethanolamine/

