Domestic researchers have made new progress in bio-based recyclable polyurethane
Latest News:
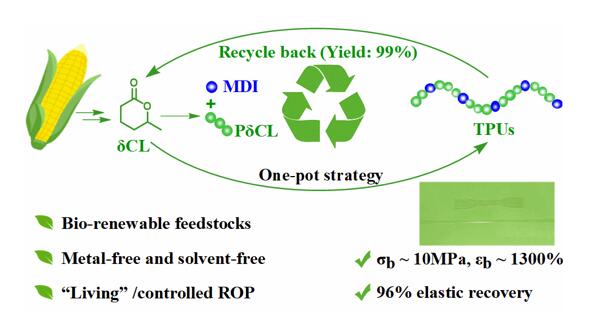
Polymer materials have become an integral part of our daily life, but their mass production and disposal also pose serious resource and environmental problems. It is imperative to develop sustainable polymer materials to meet current challenges. The best solution to the problem of contamination of polymeric materials is to develop chemically recyclable polymers with closed-loop life cycles, which can be recycled to the starting monomers or converted to high-value chemicals after the end of their useful life. As an important polymer material, polyurethane is widely used in construction, furniture, textiles, automobiles, electronics and other fields, with an annual output of tens of millions of tons. But most commercial polyurethanes have long degradation cycles under natural conditions, so their mass production and disposal pose significant environmental challenges. At present, the chemical recovery of polyether polyol is mainly realized by alcoholysis or hydrolysis of polyurethane. However, the disadvantages of this method are the low purity of the recovered polyether polyol, the need to use a large amount of solvent, poor selectivity and low recovery efficiency. Therefore, there is an urgent need to synthesize chemically recyclable polyurethane materials from biorenewable raw materials and realize their efficient and selective chemical recovery under mild conditions.
The team of Shen Yong and Li Zhibo from Qingdao University of Science and Technology used a series of ring-opening polymerization/polycondensation reactions to prepare thermoplastic polyurethane elastomers with excellent properties in a one-pot method under solvent-free conditions. It is worth noting that in the presence of catalyst, the prepared polyurethane elastomer can be recovered by vacuum distillation to obtain high-purity δ-caprolactone (δCL) monomer (yield ∼99%).
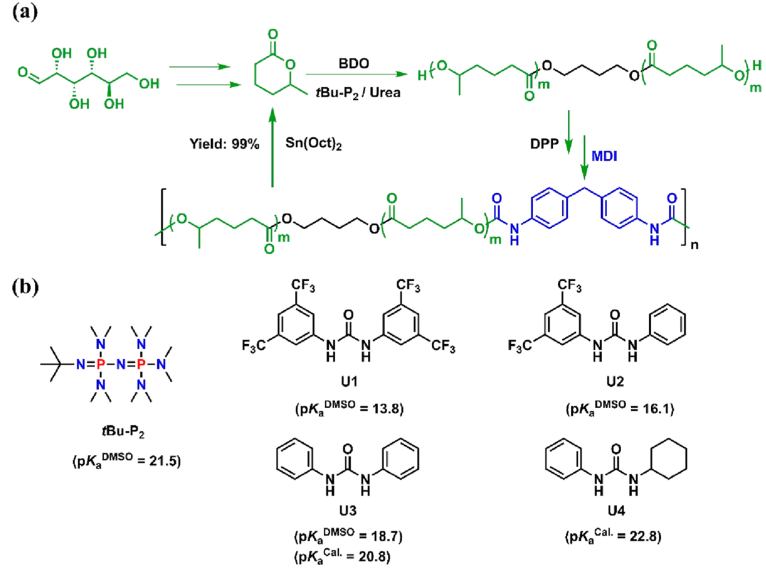
Figure 1. (a) Preparation of chemically recyclable polyurethanes using biobased δCL; (b) chemical structures of organic bases and ureas
The authors successfully realized the “living”/controllable ring-opening polymerization of bio-based δ-caprolactone (δCL) under bulk conditions at room temperature by screening suitable organic bases and urea to form a binary catalytic system, and obtained molecular weight and end groups. Controlled poly(δ-caprolactone) (PδCL) polyols (Figures 1 and 2). even at 0.05
The catalytic system also exhibited high catalytic activity at low concentrations of mol % (Fig. 3).
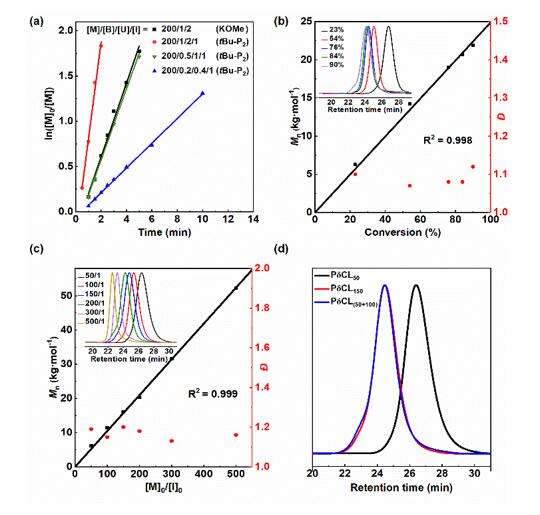
Figure 2. “Living” controllable ring-opening polymerization of δCL
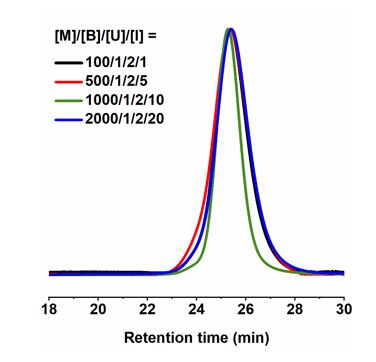
Figure 3. GPC curves of PδCL at different catalyst concentrations
Furthermore, using a catalyst switching strategy, the authors successfully prepared thermoplastic polyurethane elastomers with excellent elastic recovery, tensile strength, elongation at break, and low residual strain by a one-pot method under solvent-free conditions. The authors then investigated the chemical recycling properties of the resulting polyurethane. Using stannous octoate as a catalyst, the prepared polyurethane can be recovered as high-purity δCL by vacuum distillation at 180 °C (yield ∼99%). The recovered δCL can be repolymerized with no difference from the initial monomer (Fig. 4).
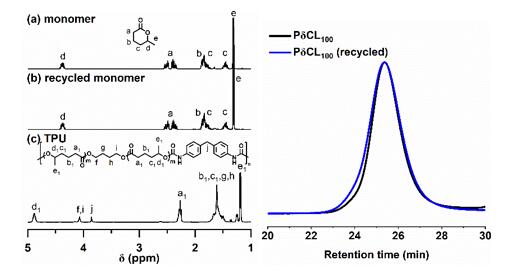
Figure 4. (Left) 1H NMR spectra of pristine δCL, recovered δCL, and polyurethane; (right) GPC curves of PδCL prepared from pristine δCL monomer (black line) and recovered monomer (blue line).
The authors realized the “living”/controllable ring-opening polymerization of bio-based δCL under bulk conditions at room temperature using an organic base/urea binary catalytic system. Chemically recyclable polyurethane elastomers with excellent properties were prepared under the conditions. This study provides a new strategy for constructing recyclable polymer materials using commercial, bio-based monomers. In the future, developing bio-based polyurethanes with excellent properties and realizing their full component recovery remains a formidable challenge.
The relevant paper was published on Macromolecules. Yan Qin, a master student of Qingdao University of Science and Technology, is the first author of the paper, and Associate Professor Shen Yong and Professor Li Zhibo of the School of Chemical Engineering are the corresponding authors of the paper. This work was supported by the National Natural Science Foundation of China, the Shandong Taishan Scholars Talent Project and the 111 Program.

